Time:2020-04-14 Reading:17889
Recently, an article titled "Current and future role of Haber-Bosch ammonia in a carbon-free energy landscape" was jointly published in the esteemed energy journal, Energy & Environmental Science, by Alfred K. Hilla from the University of Bath's Department of Chemical Engineering and Collin Smithb and Laura Torrente-Murcianob from the Department of Chemical Engineering and Biotechnology at the University of Cambridge. The abstract of the article provides a concise overview of the challenges and opportunities facing catalytic ammonia synthesis:
"In fact, replacing fossil fuels (such as methane) with electricity as a fuel and raw material will lead to significant energy efficiency improvements through the use of high-efficiency motors and the elimination of CO2 emissions. Although electrically driven Haber-Bosch ammonia synthesis is technically feasible, there remains uncertainty over whether this shift will occur. We found its success hinges on two factors: 1) enhanced energy efficiency and 2) the development of small-scale, distributed, and flexible processes that can be combined with geographically isolated, intermittent renewable energy sources. The former requires not only higher electrolytic hydrogen production efficiency but also an integrated ammonia synthesis cycle that replaces condensation separation steps with absorption and catalytic development. These innovations pave the way for moderate pressure systems, the development and application of novel ammonia synthesis catalysts, and, more importantly, opportunities to integrate reaction and separation steps to overcome equilibrium limitations. Once achieved, green ammonia will reshape the current energy landscape by directly replacing fossil fuels in transportation, heating, and electricity sectors."
Based on the authors' perspectives, the future clean energy market will undergo localized development due to geographical factors (distribution of solar, wind, tidal, and geothermal energy). They envision that the future renewable energy market will mirror the current fossil fuel market, with many nations inevitably becoming net energy importers or exporters. In this new energy landscape, ammonia offers a unique opportunity, given its high hydrogen content and a mature industrial foundation.
Addressing the substantial greenhouse gas emissions stemming from the Haber-Bosch process, researchers have proposed several optimization strategies. It's important to emphasize that the current Haber-Bosch process evolved against a backdrop where fossil fuels were seen as the only feasible energy source, leading to misguided optimizations tailored to the inefficiency of hydrogen production from fossil fuels, such as methane.
While it's known that the Haber-Bosch reaction does not involve carbon, to genuinely realize carbon-free ammonia production, the following technological changes need to be implemented:
Decoupling it from the methane reforming process.
Replacing condensation steam turbine compressors with electric compressors.
Using alternative ammonia separation techniques to reduce operational pressure.
From an industrial perspective, there's a need to establish small-scale production systems that are in sync with the intermittency and geographical isolation of renewable energy generation. The realization of a carbon-neutral industrial process for Haber-Bosch hinges on two key technologies:
1) A more efficient water electrolysis process, and
2) A simplified Haber-Bosch process that incorporates milder reactions and separations.
These two sectors present promising opportunities, aligning ammonia with renewable energies, reshaping its 20th-century role as a fertilizer, and pioneering its 21st-century role as a hydrogen and energy storage medium.
Comparison of Old and New Technologies
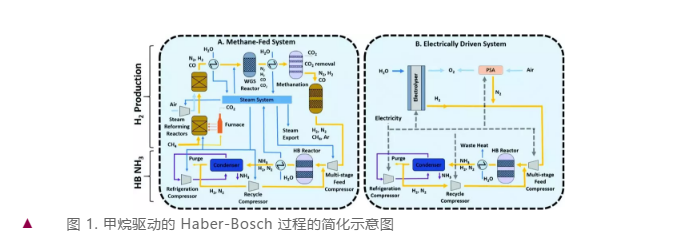
Figure1 A simplified schematic diagram of the methane-driven Haber-Bosch process.
As illustrated in Figure 1a, the process is highly integrated, comprising two primary steps: hydrogen production from methane and ammonia synthesis via the H-B method. This encompasses various reactions like methane reforming, steam compression, WGS (Water Gas Shift), and more. With electricity offering a more versatile mode of application, renewable energy has the potential to meet all energy requirements, replacing methane as both a feedstock and fuel. Hydrogen can be generated through water electrolysis, and similar to the traditional process mentioned above, the H-B reaction can convert it to ammonia. Figure 1b outlines a generic procedure where N2 is transported via Pressure Swing Adsorption (PSA), serving as a starting point for process development. Future advancements should consider alternatives like low-temperature distillation (suitable for large-scale operations) and membrane separation (assuming the required N2 purity can be achieved).
The concept of electrically driven ammonia synthesis isn't a new
proposal. However, it hasn't been widely adopted because most of the
electricity is still derived from fossil fuels. But with the growth and
utilization of clean energy, electrically driven ammonia synthesis will
undoubtedly showcase its unique advantages.
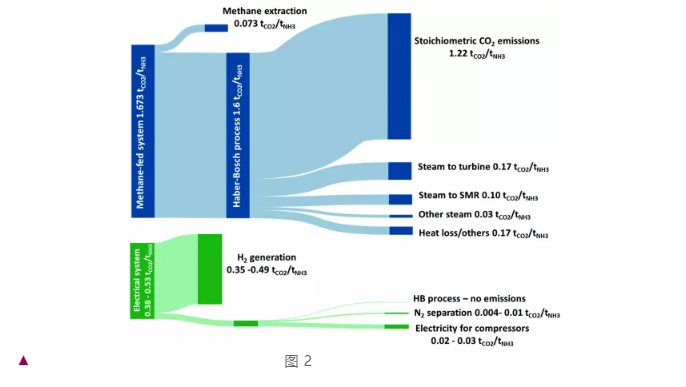
In this section, the author primarily evaluates the feasibility of electrically driven ammonia synthesis by considering energy density and relative emissions. In Figure 2, item 68 mainly displays the relative CO2 emissions between the traditional methane-driven ammonia synthesis process and the new electrically driven ammonia synthesis process. It's evident that in the traditional process, the majority of carbon dioxide emissions comes from the carbon in the reactant—methane (since the hydrogen in ammonia comes from methane). Meanwhile, the carbon dioxide emissions of the new synthesis strategy mainly arise from the hydrogen evolution and separation process (the emissions equivalently derived by the author through electrolytic energy), and this portion will gradually reduce as the electricity becomes cleaner.
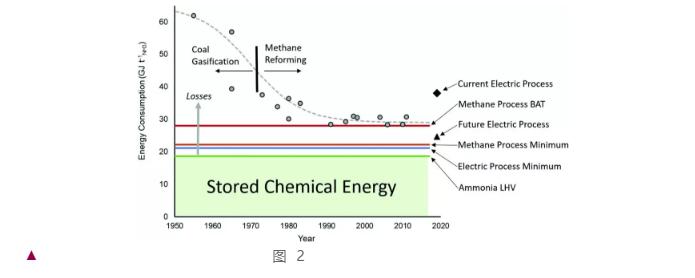
Additionally, the author places special emphasis on the “energy efficiency” of the production process, i.e., the ratio between the energy consumed per ton of ammonia and the heat of combustion of ammonia (18.6 GJ/tNH3). As depicted in Figure 3, with the gradual optimization of the H-B process and the maturation of gas separation technology, fossil-fuel-driven ammonia synthesis has approached the theoretical limit of energy efficiency (the energy lost here is roughly equivalent to the energy consumption of the Rankine cycle). To surpass this limit, it's essential to adopt and further refine electrically driven ammonia synthesis, which includes the preliminary electrically driven hydrogen production and the separation of H-B tail gas. After a comprehensive evaluation and research on the current synthesis process, the author estimates that the electric H-B process can improve the overall energy efficiency by 50%, reduce CO2 emissions by 78%, and the by-products CO2 and O2 can also be used in the urea synthesis industry and medical treatments. The possibility of further implementing the Allam cycle [1] is also discussed.
PS: [Personal Note from the Writer] The writer believes that it's worth introducing the concept of energy efficiency into the full water splitting as well as emerging electrocatalytic nitrogen and carbon fixation works to benchmark industrial production. While most of the full water splitting works emphasize water splitting voltage, the writer feels that energy efficiency can be calculated by measuring the hydrogen production and electricity consumption (for instance, achieving a 1.5 V full water splitting current of 10 mA can reach 98% energy efficiency under ideal Faraday efficiency conditions). Similarly, in nitrogen and carbon fixation catalysis, maximum yield and efficiency potential (over-potential) should be referenced to judge whether an electrocatalyst can effectively enhance the energy efficiency of catalytic conversion.
Potential for Industrial Implementation
In this section, the author mainly discusses the potential and developmental space for the industrial implementation of the new electrically driven ammonia synthesis strategy. As the core of the electrically driven process, the author benchmarks currently commercially available alkaline electrolyzers, PEM electrolyzers, and SO electrolyzers. He lists the development and optimization of electrolysis cells and H-B catalysts since the 1950s. The conclusion drawn is that the current H-B cycle is limited by ammonia separation technology, and future innovations should focus on using absorption to replace condensation to achieve ammonia separation in the H-B cycle.
Lastly, the author also discusses other ammonia synthesis strategies. Direct electrochemical synthesis of ammonia using H2O and N2 as raw materials is seen as a promising alternative and has even begun entering the industrial market. However, it faces significant challenges in selectivity and yield, requiring further research and improvement. In the studied transition metal electrodes, the minimum potential for nitrogen reduction always falls below that for hydrogen evolution. Hence, hydrogen evolution always occurs preferentially at the electrode surface, exacerbating the issue of selectivity. Even though recent reports have highlighted efficient electrocatalytic ammonia synthesis catalysts, their energy consumption remains about double that of the traditional process (even if the theoretical energy consumption is only 60% of the conventional process). Analogous to electrocatalytic synthesis, photocatalytic ammonia synthesis utilizes light-generated potentials on semiconductors or plasmonic materials to fix nitrogen. However, these catalysts are still at the experimental stage.
Conclusion
By using renewable energy to replace the carbon-intensive methane hydrogen production process with water splitting, a second ammonia revolution in a carbon-free economy becomes feasible, substantially reducing CO2 emissions. Separating H2 production from the ammonia synthesis cycle suits the inefficiency of methane steam reforming brought by the low prices and high availability of natural gas. In the realm of new energy, electrically driven Haber-Bosch can enhance the energy efficiency of the synthesis cycle by about 50% (4.2 GJ/tNH3). Enhancing water-splitting efficiency, refining ammonia separation technology, and developing catalysts are pivotal areas requiring further research and advancement. The feasibility of electrification depends on the design of its synthesis system, construction costs, and its capability to utilize isolated and intermittent renewable energy sources. Sustainable ammonia production will liberate developed nations from fossil fuels and promote growth in developing countries, subsequently alleviating poverty. The uniqueness of ammonia lies in its role not only as a prerequisite for current human food supply but also as a key for humans to fully tap into renewable energy resources in the future.
References
[1] R. Allam, S.
Martin, B. Forrest, J. Fetvedt, X. J. Lu, D. Freed, G. W. Brown, T. Sasaki, M.
Itoh and J. Manning, in 13th International Conference on Greenhouse Gas Control
Technologies, Ghgt-13, eds. T. Dixon, L. Laloui and S. Twinning, 2017, vol.
114, pp. 5948-5966.
The article is
sourced from the official account RSC (Royal Society of Chemistry, UK).