Time:2020-04-20 Reading:17638
Hydrogen, methane, and other energy gases are vital and eco-friendly energy carriers for people today. However, the optimal storage of these gases poses a considerable constraint on their use.

[Image caption] Screenshot of the research
paper on the "Science" magazine official website. (Source: Science)
Why hydrogen and methane?

(Source: DeepTech)
MOFs (Metal-Organic Frameworks) materials are composed of organic molecules and metal ions or clusters. The organic molecules usually contain elements such as carbon, oxygen, nitrogen, and phosphorus, most of which are aromatic carboxylic acids. They form multi-dimensional, highly crystalline, porous frameworks through self-assembly. The earliest prototypes of MOFs can be traced back to materials like Prussian Blue.
"To visualize what a MOF is, you can imagine it as a set of Tinker Toys (circuit blocks similar to LEGO)," Omar explained to DeepTech. "Here, metal clusters or ions can be considered as the circular or square nodes of the blocks, while organic molecules are the connecting rods."
Omar’s research group has been studying how "atomically precise location" functional materials can solve exciting problems in chemistry and materials science, with application areas covering energy and the environment. They are committed to fundamentally understanding the role of three-dimensional structures in altering material functions, applying them to gas storage and separation, catalysis, and water pollution treatment.
"In this latest research, we synthesized a MOF called NU-1500, based on past works. We used six organic linkers, combined with metal trimers of iron, aluminum, chromium, or thulium to construct NU-1500," Omar explained. These initial materials all showed good gas adsorption properties. However, their pore sizes and volumes are relatively small, limiting their weight performance.
"The relationship between volumetric and gravimetric gas adsorption of these previously studied materials inspired our contact with collaborators. They can calculate the relationship between the structures and properties of MOF-like materials," Omar said.
Ryther Anderson from the Colorado School of Mines simulated the gas adsorption behavior of many MOFs with similar topological structures, pore sizes, and organic connectors. From their work, Omar's team discovered a new type of MOF that had never been synthesized before, anticipated to have an ideal balance of weight and volume for methane and hydrogen gas storage performance. They then synthesized this MOF in the lab and measured its storage capacity for methane and hydrogen under various conditions.
Dr. Penghao Li, a postdoc under Sir Fraser Stoddart, was responsible for the synthesis of the organic ligands. It's worth mentioning that Sir Fraser Stoddart, along with two other scientists, developed molecular machines, opening a new world for the development of chemistry by winning the Nobel Prize in Chemistry. These molecular machines have now been used in the research and development of new materials, novel sensors, and energy storage systems.
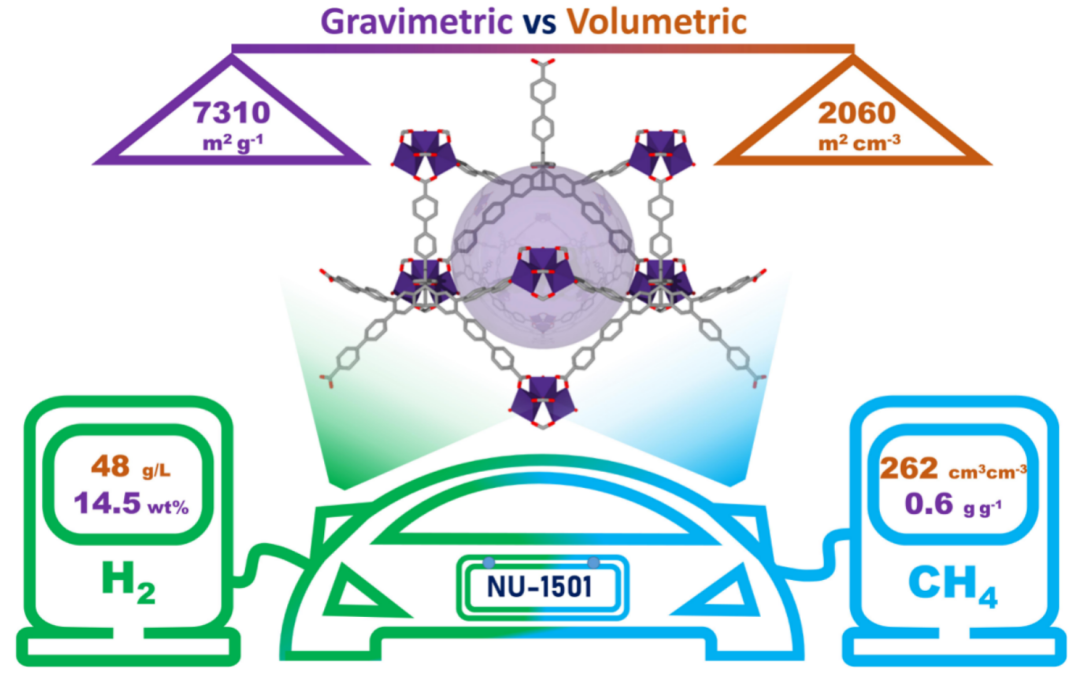
Image | Organic-Inorganic Hybrid Porous Material with Extremely High Porosity and Surface Area (Source: Timur Islamoglu & Chen Zhijie)
Regarding the synthesis method, Chen Zhijie stated that they used a solvent-thermal synthesis method to prepare this MOF—putting metal salts and organic linkers into a sealed container and baking it in a preheated furnace. "The material is activated by supercritical carbon dioxide, a common method we developed for activating MOFs with extremely high porosity and surface areas," he said.
So, is it possible for this seemingly magical material to be mass-produced in the short term? Omar said that the current laboratory research focuses more on academically enhancing the basic understanding of such materials, and developing a knowledge base of their unique structural and property relationships. "However, from a commercial perspective, we believe that the most significant material cost for scaling up this special MOF comes from the synthesis of the organic ligands," he said. "This is because the metal trimers are based on cheap, and abundantly available metals like aluminum and iron. Of course, introducing a solvent recovery system could significantly reduce the cost of organic solvents used in the synthesis process." Omar believes that these materials are very likely to be synthesized on a large scale commercially in the future. "I hope this research can be applied in the next few years," he said. "I believe that the first field to be entered will be the natural gas storage industry. In fact, there are already commercial applications using some MOFs with ION-X cylinders for storing toxic gases at relatively low compression pressures."
In his view, the safety optimizations brought by MOF materials will change the way gases are stored, transported, and conveyed. "This is a study that might change the entire gas storage industry," Omar stated.
Main researchers and lead authors introduction
Image description | On the left is Dr. Chen
Zhijie; on the right is Dr. Li Penghao (Source: Personal)
Chen Zhijie is currently conducting
postdoctoral research in the chemistry department at Northwestern University in
Omar Farha's laboratory. He began his studies at Shanghai Jiao Tong
University's School of Chemistry and Chemical Engineering in 2008. After
obtaining his bachelor's degree in 2012, he went to King Abdullah University of
Science and Technology (KAUST) in Saudi Arabia for his doctoral studies. Since
obtaining his Ph.D. in 2018, he has been conducting postdoctoral research at
Northwestern University.
Since August 2017, he has been conducting postdoctoral research at Northwestern University. His main research topics include the synthesis of organic porous materials as well as their topological research and characterization.
-End-
References:
https://science.sciencemag.org/content/368/6488/297
https://sites.northwestern.edu/omarkfarha/
https://sites.northwestern.edu/omarkfarha/group-members-2/
https://www.in-en.com/article/html/energy-2283805.shtml
https://www.nobelprize.org/prizes/chemistry/2016/stoddart/facts/
The article is sourced from the WeChat
Official Account 'DeepTech深科技'.