Time:2020-05-20 Reading:19739
Thermoplastic elastomers (TPE) are a class of
high-molecular materials that can be easily molded, extruded, and reused like
plastics, while also possessing the typical elastic properties of rubber. They
are widely used in industries such as automotive and home appliances. The idea
of synthesizing thermoplastic polyolefin elastomers from ethylene in a single
step is both intriguing and challenging. In recent years, Professor Chen
Changle's research group at USTC (University of Science and Technology of
China) and some colleagues have successfully synthesized polyethylene
thermoplastic elastomers via α-diimine nickel-catalyzed ethylene
polymerization, achieving a series of significant results. Since the α-diimine
palladium catalyst tends to have greater chain walking than nickel catalysts,
it often results in highly branched polyolefins with inferior mechanical
properties, making its use in the preparation and application of thermoplastic
polyolefin elastomers very challenging. In this work, they have, for the first
time, reported the direct synthesis of polyethylene thermoplastic elastomers
catalyzed by an α-diimine palladium catalyst. With precise catalyst design and
polymerization adjustment, high-performance polyethylene thermoplastic
elastomers can be produced (with an elasticity recovery value reaching up to
83%). Most importantly and significantly, polar functionalized polyethylene
thermoplastic elastomers can be prepared through the copolymerization of ethylene
with biomass-derived comonomers, and the resultant polar functionalized
polyethylene thermoplastic elastomers also exhibit excellent elastic properties
(with an elasticity recovery value reaching up to 80%).
In this study, the research team first prepared a diimine palladium catalyst substituted with a large steric hindrance tertiary butyl group, labeled 1-2. It demonstrates moderate polymerization activity in ethylene polymerization, producing polyethylene with a high molecular weight (Mn up to 45.58 x 104) and a medium branching structure (60/1000C). At the same time, using the reported bulky palladium catalysts 3-7 under the same conditions, they synthesized a series of high molecular weight polyethylenes with varying branch structures (shown in
Figure 2). Additionally,
they utilized catalysts 1-2 to prepare high molecular weight (Mn up to 34.09 x
104) medium branched biomass-functionalized polyethylene, which
retains high activity during copolymerization.
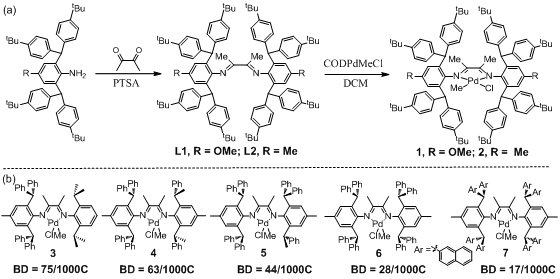
Figure 2. Diimine palladium catalysts prepared in this
study with tertiary butyl substitution and a series of reported bulky diimine
palladium catalysts.
The polymers obtained from the aforementioned catalysts
were first subjected to tensile strength tests (Figure 3). These samples showed
mechanical properties characterized by relatively low break stress values
(3.3-17.6 MPa) and very high strain-at-break values (452% - 1841%). Typically,
under the same other conditions, the breaking stress and Young's modulus
increase with increasing ethylene pressure. This indicates that increasing the
melting point of the polymer or reducing its branching density will increase
the material's ultimate tensile strength and tensile toughness. Meanwhile,
under the same other conditions, polyethylene obtained from catalyst 1 has
higher ultimate tensile strength and tensile toughness than that obtained from
catalyst 2. This is mainly because the electron-rich catalyst 1 can produce
polyethylene with a lower degree of branching and a higher melting point. The
aforementioned experiments show that by merely altering the polymerization
conditions or modifying the electronic effects of the ligands, the mechanical
properties of the resulting polyethylene can be adjusted. For comparison, they
examined polyethylene produced by catalysts 4-6, which displayed very high
breaking stress values (19.6-26.0 MPa) and high strain-at-break values (500% -
860%). This suggests that polymers prepared by catalysts 1-2 have better
elastomeric potential. They further studied the elasticity recovery rate of the
resulting polymers (strain lag tests).
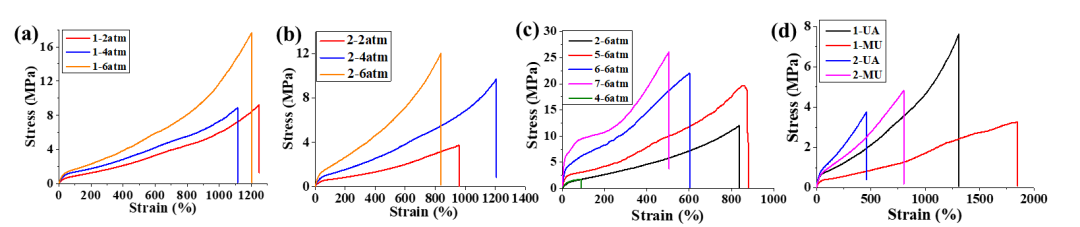
Figure 3. Stress-strain curves of the prepared polyethylene
and polar copolymers.
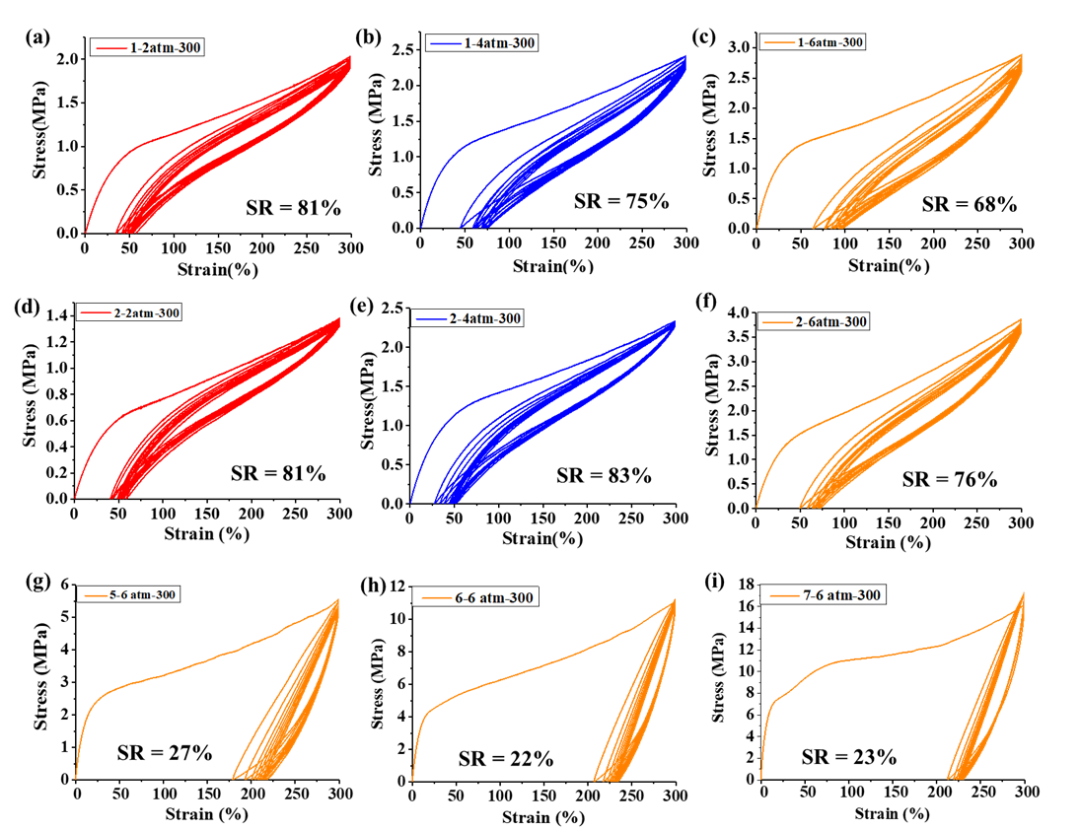
Polyethylene prepared using catalysts 1-2 had an elasticity
recovery rate (SR) of 68% to 83%, comparable to previously reported
polyethylene elastomers obtained through α-diimine nickel catalysts (Figure 4).
In contrast, polyethylene produced by catalysts 5-7 displayed inferior elastic
properties, with SR values of 22% -27% (Figure 4). The polymer's high molecular
weight and the appropriate high branching microstructure (high entropic
elasticity and suitable crystalline ratio) seem to be the primary factors
contributing to their excellent elasticity. Thus, the catalytic system reported
in this study offers an alternative effective route for the synthesis of
thermoplastic polyethylene elastomers. Most importantly, to the best of our
knowledge, this is the first time thermoplastic polyethylene elastomers have
been prepared via an α-diimine palladium system, with most previous preparations
coming from nickel systems. Interestingly, biomass-functionalized polyethylene
prepared by catalysts 1-2 also has excellent elastic properties (SR = 72% -80%)
(Figure 5). The above experimental results indicate that the structure of the
catalyst plays a decisive role in the elastic properties of these polymer
samples.
The related research was published under the title
"Direct Synthesis of Polar Functionalized Polyethylene Thermoplastic
Elastomer" in the journal Macromolecules (2020, 53, 2539-2546). Professor
Dai Shengyu of Anhui University and Professor Chen Changle of the University of
Science and Technology of China were the corresponding authors. This work was
funded by the National Natural Science Foundation of China (NSFC 51703215, 21690071, U19B6001, and
U1904212).
Reference link:
https://pubs.acs.org/doi/10.1021/acs.macromol.0c00083