Time:2020-08-20 Reading:15226
▲First authors: WEI Qinhong, LI Hangjie,
LIU Grasshopper; Correspondence should be addressed to CHUN Fanli, YANG Guohui,
PENG Xiaobo
Correspondence should be addressed to
National University of Toyama, Japan, Shanxi Institute of Coal Chemistry,
Chinese Academy of Sciences, National Institute for Materials Research, Japan,
and Zhejiang Normal University, China.
Paper DOI: 10.1038/s41467-020-17941-8
Full text brief
Recently, a collaborative team of Professor
Noritatsu Tsubaki of National University of Toyama, Japan, Researcher Yang
Guohui of Shanxi Institute of Coal Chemistry, Chinese Academy of Sciences and
Dr Peng Xiaobo of National Institute for Materials Research (NIMR), Japan, has
successfully achieved the design and construction of an autocatalytic reactor
coupled with catalysts and reactors through metal 3D printing technology. The
design breaks the convention by not requiring a catalyst to be loaded inside
the tube. The iron-based, cobalt-based and nickel-based 3D autocatalytic
reactors it developed not only have the ability to withstand high temperature
and high pressure, but also assume the role of catalysts, and show extremely
broad catalytic applications in typical C1 reactions such as Fischer-Tropsch
Synthesis, CO2 Hydrogenation, and Dry Reforming of Methane. In addition, the
theory of morphology control of 3D autocatalytic reactors opens up new research
directions for future autocatalytic synthesis. This work, entitled "Metal
3D Printing Technology for Functional Integration of Catalytic System",
was recently published in Nature Communications, and has been applied
for international publication. This work has been applied for an international
patent.
Research background
Catalysts and reactors are two of the most
important elements in catalytic reactions. However, for a long time the design
of catalysts and reactors have always been developed relatively independently
in their own fields. The integrated coupling and synergy of catalysts and
reactors are also rarely reported. 3D printing, as an additive mode of making
things, has shown a strong development trend in biotechnology, pharmaceuticals,
and mechanical manufacturing, but its development in the chemical and chemical
fields is very slow. Metal 3D printing, as an important branch of 3D printing
technology, has inherent advantages for the integrated coupling of catalysts
and reactors:
(1) The metal itself has catalytic ability
and is resistant to high temperature and pressure.
(2) Energy transfer efficiency, much higher
than conventional catalytic reaction systems.
(3) Eliminate the need for binder moulding
of conventional solid catalysts and improve catalyst stability.
(4) Computer controlled printing to
eliminate the error of hand in catalyst and reactor fabrication.
(5) Highly flexible and free design of the
shape of the printed product.
Metal 3D printing technology, therefore,
offers a whole new way of thinking about the design of catalysts and reactors
for petrochemicals, C1 chemistry, or other catalytic reactions.
Highlights of this article
(1) This study reports, for the first time,
the preparation of autocatalytic reactors with tunable species and morphology
using metal 3D printing.
(2) An iron-based autocatalytic reactor,
which exhibits good pressure resistance and reactivity in Fischer-Tropsch
synthesis and carbon dioxide hydrogenation, and characterization experiments
have demonstrated that a catalytically active layer on the inner wall of the
reactor is essential for the reaction to proceed.
(3) Cobalt-based autocatalytic reactor
exhibiting good liquid fuel selectivity in Fischer-Tropsch synthesis.
(4) Nickel-based autocatalytic reactor
showing excellent high temperature resistance and catalytic performance in the
reaction of carbon dioxide reforming methane.
(5) Conformal studies of autocatalytic
reactors demonstrate that the highly free design of 3D printing can modulate
the catalytic function of the reaction system.
graphic resolution

▲ Figure 1. 3D printing for self-catalytic
reactor (SCR) and other typical applications. The inset: (left) SCR for
Fischer-Tropsch synthesis; (middle) SCR for CO2 hydrogenation; (right) SCR for
CO2 reforming of CH4. (left) SCR for Fischer-Tropsch synthesis; (middle) SCR
for CO2 hydrogenation; (right) SCR for CO2 reforming of CH4.
3D printing is an additive manufacturing
technology that has been extensively researched in biotechnology, prosthetics,
pharmaceuticals, and mechanical engineering (Figure 1). Metal 3D printing
technology, was applied in this study. The technology uses metal powder as raw
material to achieve rapid prototyping of autocatalytic reactors by
layer-by-layer printing. The printed iron-based Fe-SCR, cobalt-based Co-SCR and
nickel-based Ni-SCR autocatalytic reactors can be applied to catalytic
reactions such as Fischer-Tropsch synthesis, carbon dioxide hydrogenation and Dry
Reforming of Methane, respectively.
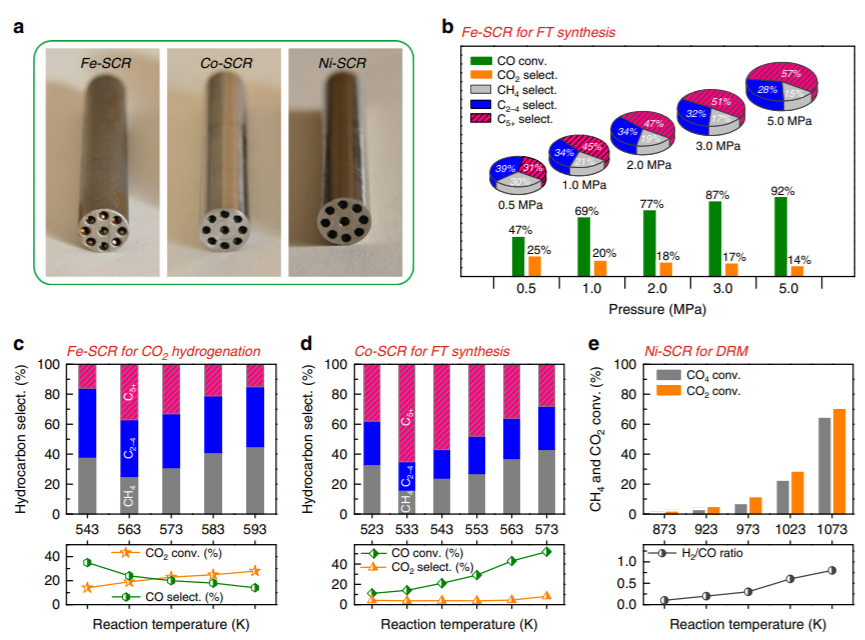
Figure 2. Catalytic performance of SCRs. a,
The physical SCRs after polishing the outer surface.
b, Fe-SCR for Fischer-Tropsch synthesis. c, Fe-SCR for CO2 hydrogenation. d, Co-SCR for Fischer-Tropsch synthesis. e, Ni-SCR for CO2 reforming of CH4. CO2 hydrogenation. d, Co-SCR for Fischer-Tropsch synthesis. e, Ni-SCR for CO2 reforming of CH4.
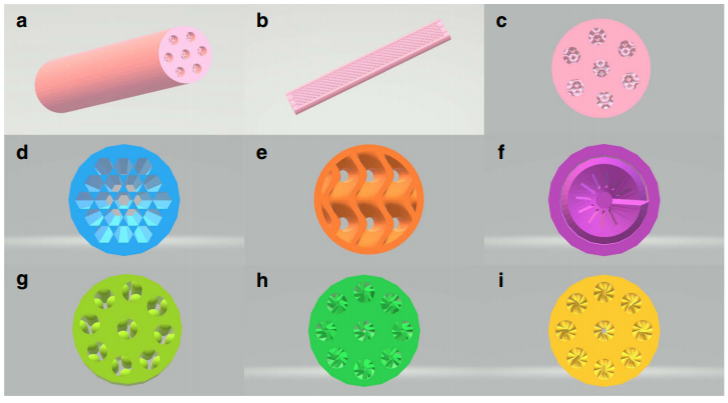
▲ Figure 3. Geometrical structures of
Co-SCRs. a, Co-SCR-1; b, longitudinal section of Co-SCR-1; c, cross-section of
Co-SCR-1; d, cross-section of Co- SCR-2; e, cross-section of Co-SCR-3; f,
cross-section of Co-SCR-4; g, cross-section of Co-SCR; h, cross-section of
Co-SCR-5; i, cross-section of Co- SCR-6.
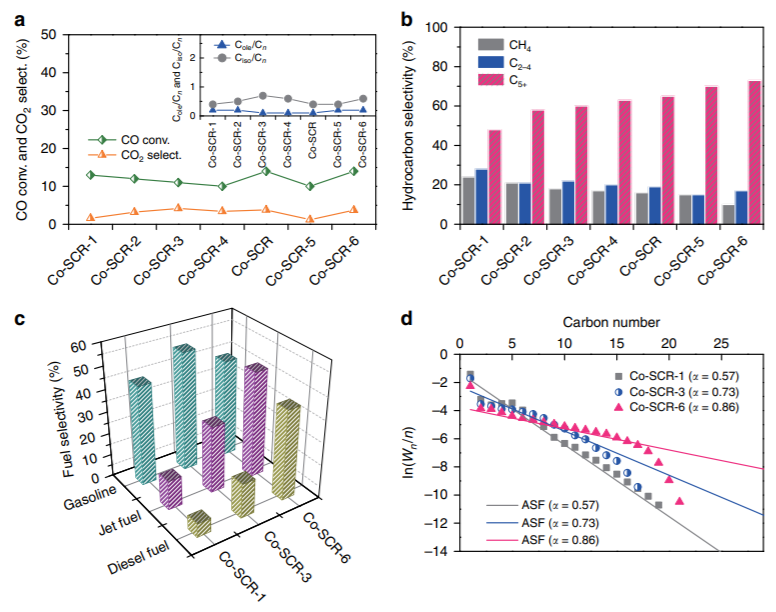
▲ Figure 4.